Hydrolysis is a chemical reaction in which water breaks bonds between two substances. It is a process used in solvation, elimination, and substitution reactions. Water is the nucleophile in hydrolysis. It also occurs in the biochemical reactions of enzymes, bacteria, and fungi. It is an important chemical reaction in many biochemical processes, including biotechnology.
Saponification
The process of hydrolysis for saponification involves converting a cyclic ester into an acyclic hydroxy-acid. This is done by adding a hydroxide ion to the ester. It is very similar to the intermolecular process, as the bonds are formed and broken in the same way. The term “saponification” derives from the word “lain sapo”, which means “soap”. The process is performed on the racemic a-hydroxy-methyl ester. Aqueous lithium hydroxide was used to hydrolyze the ester in multigram quantities. This procedure has been used to synthesize a-hydroxy-a-substituted carboxylic acids in good to moderate yields and diastereoselectivity.
In nature, fatty acids are rarely free molecules. They are usually a part of a triglyceride, a three-membered carbon chain with fatty acids attached to each carbon atom. The fatty acid in triglycerides is bonded to the backbone of the glycerol molecule by a chemical bond called an ester. During the saponification process, the ester linkage between the fatty acid and glycerol breaks down. The resulting soap is usually used for cleaning and other applications.
Acid hydrolysis
Acid hydrolysis is an important process for the production of valuable chemicals from biomass. It was originally developed to produce ethanol but has since been used to produce a variety of other chemicals. The process works by breaking down the cellulosic fiber matrix and converting the polysaccharides present in the fiber to monosaccharides. This process can be performed at either a high or low temperature.
Hydrolysis is a process in which a weak acid or base reacts with water to form a more reactive compound. The acid cleaves the chemical bonds in the molecule and the water transforms them into monomers. The hydrogen and hydroxyl ions are responsible for catalyzing the reaction. While acid hydrolysis is typically carried out in acidic environments, it can also occur in a neutral pH environment. This process is used to produce alcohols, glycols, and propylene oxide.
Base dissociation
Base dissociation during hydrolysis is a reaction in which one acid dissociates from another acid. While the equilibrium constants of acid dissociation and base hydrolysis are similar, the ionic charges of the two ions are different. This is an important consideration when comparing acids and bases.
Hydrolysis occurs when a weak acid or base reacts with water to break down its bonds. The water molecules are then spontaneously ionized, forming hydroxide anions and hydronium cations. In addition, the salts dissociate into their constituent ions. For example, sodium acetate dissolves into sodium and acetate ions. These ions react with the hydroxide and hydronium ions only slightly, causing the compound to dissolve in water.
The reaction between base A-(aq) and water is called hydrolysis. If the acid is weak, the reaction will result in the formation of HF (s), while if the acid is strong, it will lead to the formation of Na+ (aq). Hydrolysis will therefore be complete, but incomplete for weak acids. Nevertheless, the equilibrium constant between acid and base dissociation, known as Kw, is equal to 1.0×10-14.
Nucleophile
Hydrolysis of nucleophile involves the removal of an atom from a molecule. The atom is usually a cation or a ligand. The atom can either be positively or negatively charged. The atom can act as the nucleophile or the co-solvent.
In a typical reaction, two reactants are bound by a specific geometry in the active site of an enzyme. The resulting stereochemical result is either an inversion or retention of the configuration at the electrophilic carbon. The stereochemical outcome of the reaction is dependent on the nature of the two reactants. For example, a reaction involving a charged nucleophile has a large exothermic shift compared to a reaction involving a neutral nucleophile.
Hydrolysis of nucleophiles occurs in a variety of conditions. It occurs in a variety of chemical systems, including the atmosphere, groundwater, particle-water interface in soils, and living organisms. The reactants may be neutral or ionic molecules.
Electrophile
Hydrolysis of electrophiles is a process in which a chemical compound is converted into an acid or a base. This reaction requires water as the nucleophile and releases 93 kJ/mol of thermal energy. The end product is a carboxylic acid or HCl. Dichloroacetic acid is a carboxylic acid with two chlorine atoms and a pKa of 1.26. Both substances are cytotoxic and cause localized cell death.
The first step of hydrolysis of amides involves the reversible protonation of carbonyl oxygen. This makes the carbonyl carbon a more effective electrophile. It also results in the resonance form of the carbon atom with the carbocation. Next, the nucleophile either forms a new C-O bond or breaks the old C-O pi bond.
Starch
Hydrolysis of starch is a process that makes starch into monosaccharides. The degrees of branching and the length of side chains vary among starches. The more branched the chain, the more soluble the starch is. Hydrolysis is performed at a temperature above 20 °C.
Hydrolysis is achieved by adding an acid or an enzyme to the starch. The goal of this experiment is to demonstrate that the acid or enzyme helps in hydrolyzing starch. Although not all sugars give a positive test when added to Benedict’s solution, those formed during starch hydrolysis are the ones that do.
The hydrolysis process can produce several products. Depending on the enzyme or acid used, starch hydrolyzates are classified into different types. They differ in their fermentability and their sweetening properties. Hydrolyzates with low D.E. are preferred in flavor enhancement and sweetening applications.
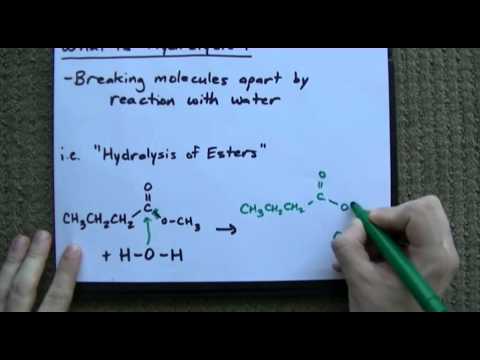
Esters
In a hydrolysis process, esters are converted into their corresponding acids. The acids are generally dibasic acids, containing three to twelve carbon atoms. This process has been used for years to create various organic acids and color materials. During the process, the esters are typically hydrolyzed in an inert atmosphere. Carbon dioxide and nitrogen can be used for the process, but carbon dioxide has the best results.
pH plays a key role in the hydrolysis of esters. A lower pH is required for the process to occur. When pH is increased, the ester hydrolysis rate increases.
Phospholipids
Hydrolysis of phospholipids occurs when water is used to break down the fatty acids in a substance. Phospholipids are molecules found in cells and are highly soluble in water. Phospholipase A is a natural enzyme that can metabolize phospholipids, increasing their functionality. Phospholipase A binds to a specific fatty acid in phospholipids, increasing their hydrolysis rate.
Hydrolysis of phospholipids can occur for a variety of reasons. It may be involved in cell signaling and signal transduction. For example, agonist-induced hydrolysis of membrane phospholipids can enhance activation of protein kinase C. This activation is critical for the long-term response of cells.
Metal salts
Hydrolysis of metal salts is a chemical process that occurs when a weak acid or base dissolves a metal salt in a liquid. Many metal ions are Lewis acids, but they may undergo hydrolysis in water to form basic salts. This occurs because these metals contain hydroxyl groups directly bound to them. As a result, the positive charge on the metal ion creates a strong attraction to water and alters the electron density of water. The result is that the water spontaneously ionizes, converting the salt into hydronium cations and hydroxide anions.
The chemical behavior of these salts is similar to that observed for acids and bases. However, the electrochemical behavior of the resulting molecules is different. In acidic solutions, the charges of the metal ion are inverted. While alkaline solutions have an ionic charge that is larger, the opposite is true for bases.
Proteins
Hydrolysis of proteins can be a useful process to create highly digestible peptides and other compounds that provide physiological and nutritional functions. This process can be carried out by a number of different methods, from enzymatic methods that utilize various sources of protease enzymes to microbial fermentation. In both cases, the end result is a product known as protein hydrolysate.
During the hydrolysis process, amino acids are removed from protein molecules. This can lead to loss of nutrients. Usually, this process takes 20 min or more, but higher temperatures can speed up the process.