Atomic structure can be described in terms of electron pairing. This process occurs when a single electron pairs with another one in an orbital. The number of electrons in an orbital is called its atomic number. Many scientists have studied the structure of atoms. Some of them have discovered how the sun’s rays and radio waves are produced. Others have studied the properties of light and how electrons move in atoms.
Thomson’s atomic model
One of the first historical scientific models of the atom was Thomson’s plum pudding model. It was proposed in 1904 before the discovery of atomic structure. The model argues that the atom is formed of a combination of smaller elements, each of which can have different properties. While this may sound a bit strange, the truth is that it’s not all that dissimilar from the atomic model that we know today.
Thomson’s atomic model had many limitations. First, it failed to explain how the electrons move inside an atom. It also did not include the idea of a nucleus. In addition, it could not account for the fact that most of the space in an atom is empty.
Another major flaw of Thomson’s atomic model is that it assumes that all chemical atoms are electrically neutral. In reality, they have positive and negative charges. Therefore, the atomic radius of a chemical atom was around 10-12 m. While atoms are electrically neutral, the Thomson model assumed that electrons have a positive charge.
While Thomson’s atomic model does not account for the properties of the electron, it does account for the structure of atoms and molecules. It was based on the observations made by scientists. The results of the experiments on the deflection of atoms were inconclusive, but Thomson’s theory pointed out fruitful research paths. This ultimately led to Bohr’s development of the quantum theory of the atom in 1913.
Bohr’s atomic model
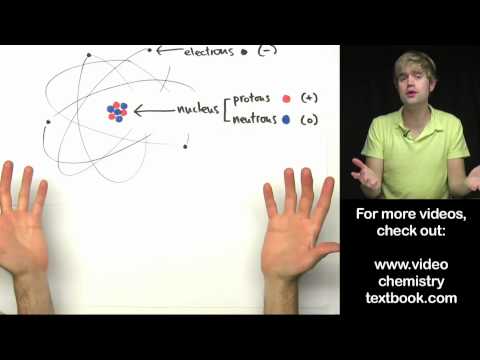
Bohr’s atomic model describes the structure of the atom. It has a dense nucleus surrounded by orbiting electrons. This structure is similar to that of our Solar System, where electrons are drawn to each other by electrostatic forces. This model also explains why stars appear to be so dense and stable.
Originally published in 1895, Bohr’s atomic model was soon improved upon by physicists based on new discoveries about particle behavior. While this model was eventually superseded by the quantum mechanical model, it remains the first thing most physics students learn about.
Bohr proposed that electrons orbit the nucleus according to certain rules consistent with Planck’s quantum theory of radiation. An electron can only occupy an allowed orbit with a certain amount of energy and must absorb or emit energy to move into another orbit. In this way, the energy of radiation emitted or absorbed by an electron is equal to the difference in the energies of the two orbits.
Despite the advantages of this model, it has numerous flaws. One of the biggest is that the atomic model does not account for microstructure and violates the Uncertainty Principle. As a result, it is difficult to predict the spectra of larger atoms based on this model. Further, the model does not account for the Zeeman effect, which is a result of the interaction between electrons.
Rutherford’s -particle scattering experiment
Rutherford’s -particle-scattering experiment first showed that protons, electrons, and neutrons are present in atoms. This discovery confirmed that the mass of an atom is mostly contained within its nucleus. This also confirmed that the electron’s charge-to-mass ratio is constant.
To test this theory, Rutherford used a thin gold foil. He then placed a fluorescent zinc sulfide screen around the gold foil. He noticed that while the majority of a-particles passed through the gold sheet without deflection, a few of them were deflected in a few angles. This is because the positive charge of atoms is not evenly distributed, but rather is concentrated in a small volume.
Rutherford’s -particle-scattering experiment gave him the answer to his question about the structure of the atom. He had predicted that positively charged alpha particles would be deflected, but he found large angles. This result confirmed his hypothesis, and allowed him to estimate the size of the nucleus. It was later discovered that the nucleus is about a ten-thousandth of an atom’s radius.
Rutherford’s experiment proved that the atom consists of a nucleus and electrons that are in the outermost shell. As a result, the nucleus of an atom is surrounded by electrons called valence electrons. Because this effect cannot be explained by Thomson’s atomic model, Rutherford was able to prove that the nucleus is present.
Atomic nucleus
The atomic nucleus consists of a group of particles called nucleons. These particles are made up of protons and neutrons. The arrangement of these particles determines the type of element. Different types of nucleon formations have different properties and are listed in the periodic table of elements.
Each particle is associated with a certain type of energy. These energies are called nuclear forces. These forces are a result of interactions between subatomic particles. In the nucleus of a molecule, the protons are positively charged and the neutrons are negatively charged. These charges attract or repel each other.
The nucleus of an atom contains all the mass of an atom, but it is tiny. The radius of the nucleus is just 1/100th the total atom’s radius. Therefore, if the nucleus were the entire atom, it would be the size of a pea. However, the neutrons and protons, which make up the nucleus, have a lot of mass for their size. In addition to their mass, the nucleus of an atom is extremely dense. If this mass were transferred to the outer space, an atom would weigh 30 million tons!
The atom’s nucleus also possesses a stationary current density distribution. This is related to the distribution of charge in the nucleus and the orbital motion of the protons. If these two properties are compatible, then the nucleus is stable.
Atomic orbits
Atomic orbitals describe the location of an electron within an atom. They describe the wave-like behavior of electrons and allow scientists to predict the probability that an electron will be located in a particular region. Atomic orbitals are also useful for calculations in physics, biology, and chemistry. For example, the probability of finding an electron in one location is increased by a factor of ten when it is paired with a positive ion.
Atomic orbitals can be represented as five-dimensional pictures. The first five atomic orbitals have radial wave structures, while the last two are spherically symmetric spheres. These shapes are the only orbitals that have an anti-node at the nucleus. All other orbitals have an angular momentum that prevents them from approaching the nucleus.
The electrons of a hydrogen atom are in elliptical orbits, which means that they take turns being near the nucleus and further away. However, this doesn’t change the fact that they feel a net repelling or attractive force from the nucleus. The electrons also exert a retarding sideways force on each other, which helps keep the electrons separated and in their orbital positions.
The atomic orbital model is a modern framework for understanding the submicroscopic behavior of electrons in matter. Atomic orbitals are defined as the regions in space around an atom where the probability of an electron being present is highest. An atom’s electron cloud is actually made up of smaller hydrogen-like atomic orbitals.
Atomic shape
Atoms are made up of a nucleus and electrons that orbit around it. The nucleus is positively charged and contains the bulk of the atom’s mass. The electrons in an atom are either protons or neutrons. Common hydrogen, for example, has one proton. All atoms are roughly the same size. The unit of length in an atom is an angstrom (A). The diameter of an atom is about two to three angstroms.
The electron cloud surrounding an atom is called an atomic orbital. When an atom has only one electron, that electron fits into its valence shell. If more electrons are present, the electron cloud tends to fill up the volume around the nucleus. This is because the uncertainty principle governs the electron cloud.
The atomic orbitals are closely related to the wave nature of electrons. Hence, the p and d modes are analogous to the s and d orbitals. The p and d modes show spatial irregularities along the radial direction, while the s mode is perfectly symmetric in this direction. This non-radial symmetry property of non-s orbitals is necessary for localizing a particle with angular momentum and wave nature. Otherwise, the angular momentum would be lost if the particle were localized at the center of attraction. In addition, the waves tend to avoid the central point.
An atom’s atomic structure can be understood using a quantum mechanical model. In quantum mechanics, electrons exist in complex shapes called electron clouds. An electron’s energy is based on probability, and the average distance of the electron from the nucleus is given by a principal quantum number called n. The higher the value of n, the more energy the electron has.